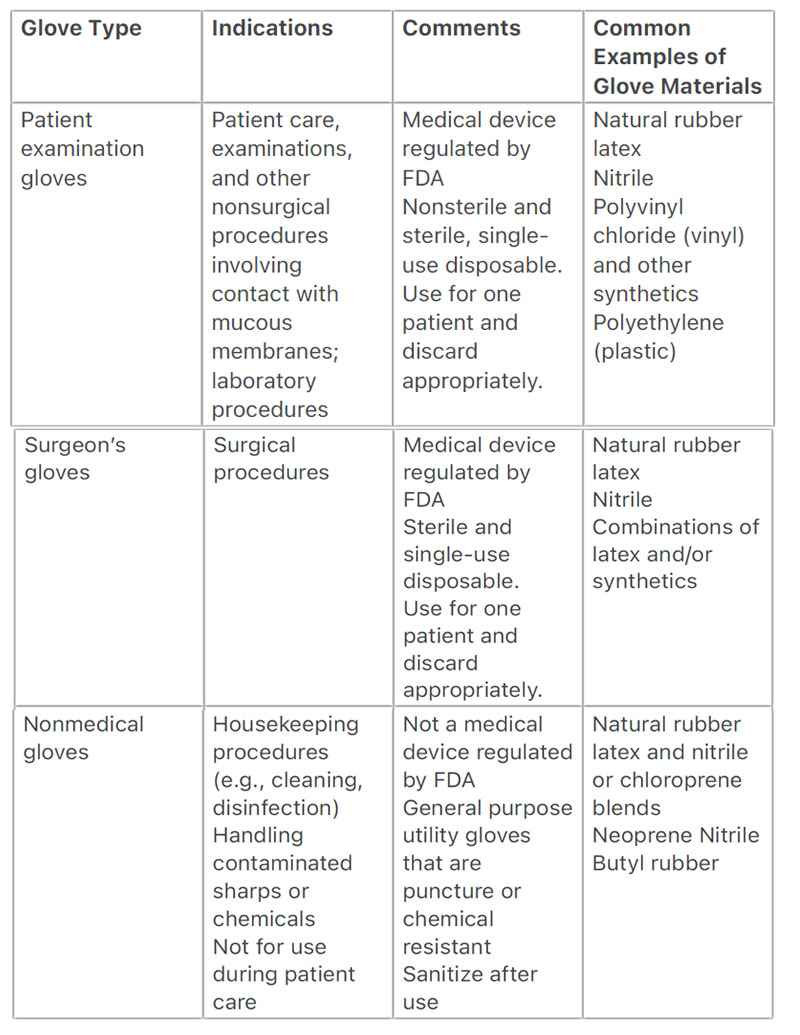
The type of glove used should be based on the type of procedure to be
performed (e.g., surgical vs. nonsurgical, housekeeping procedures). Medical-
grade non-sterile examination gloves and sterile surgical gloves are regulated
by the Food and Drug Administration (FDA) as medical devices. Sterile surgical
gloves must meet FDA standards for sterility assurance and are less likely than
non-sterile examination gloves to harbor pathogens that may contaminate an
operative wound. General purpose utility gloves are not regulated by FDA
because they are not promoted for medical use.




No comment